Lab activity : chromatography of plant’s leaves
The Sun energy fuels the metabolic processes of cells and is essential for life on earth. Plants produce glucose from the photosynthesis mechanism.
Even if Glucose is a simple carbohydrate, it provides immediate fuel to cells. Don’t forget that plants are the main source of glucose for almost all heterotrophic metabolisms !
A- Photosynthesis background and pigments chromatography
As primary producers in the food chain, plants produce their own food by using the sun’s energy to transform carbon dioxide and water into glucose. In this process of photosynthesis, plants convert the sun’s energy into chemical energy that is stored in the bonds of the glucose molecule.
For photosynthesis to transform light energy from the sun into chemical energy (bond energy) in plants, the pigment molecules absorb light to power the chemical reactions. Plant pigments are macromolecules produced by the plant, and these pigments absorb specified wavelengths of visible light to provide the energy required for photosynthesis. (Appendix A) Chlorophyll is necessary for photosynthesis, but accessory pigments collect and transfer energy to chlorophyll.
Although pigments absorb light, the wavelengths of light that are not absorbed by the plant pigments are reflected back to the eye. The reflected wavelengths are the colors we see in observing the plant. (Example: green pigments reflect green light) Plants contain different pigments, and some of the pigments observed include:
- chlorophylls (greens)
- carotenoids (yellow, orange red)
- xanthophyll (yellow)
Leaf chromatography is an experiment that allows us to see the colorful pigments that leaves have.
Questions:
- Can you remind us the process of chromatography ?
- Find on the web what does mean the ratio Rf? And Why is it interesting to study Rf for the chromatography of photosynthetic pigments?
B- Experiment
EQUIPMENT AND MATERIALS (per group)
- A spinach leaf (or equivalent – green leaf)
- A red mapple leaf (or equivalent – red leaf)
- Ruler
- Chromatography column
- Plate with paper clip to secure the paper
- Chromatography paper (whatman paper)
- Pencil
- Chromatography solvent (petroleum ether and acetone 9:1)
- Fume hood
- Glass stirrer
- Calculator
- Mortar and sand
- Pigment solvent
- Dropper
- Test tubes
- Test tube rack
- Felt pens for marking glasses

Safety advisors:
For chromatography, do not move the chromatography columns outside the fume hood and handle only with the fume hood switched on because of the petroleum ether.
Procedure:
- Take your Whatman paper and go and look under the fume hood at a chromatography column that you have made your own without moving it.
- Locate the solvent level and measure approximately how high the solvent will be on the whatman paper.
- Draw a line on the Whatman paper 2 cm above the level of the solvent
- Place a mark 1 cm from the left edge for the green leaf and a mark 1 cm from the right edge for the red leaf on the line.
- Using the glass stirrer, crush each leaf 3 times on its mark. Remember to do this on different surfaces of the plant leaf. Wait a few seconds until it dries.
- Dry each spot before the next !
- Then place the whatman paper strip in its column in a fume hood.
- observe the migration front of the eluent. When the eluent reaches the top, remove the paper strip and take a photo.
Photo: Chromatography of a yellow leaf compared to a green leaf
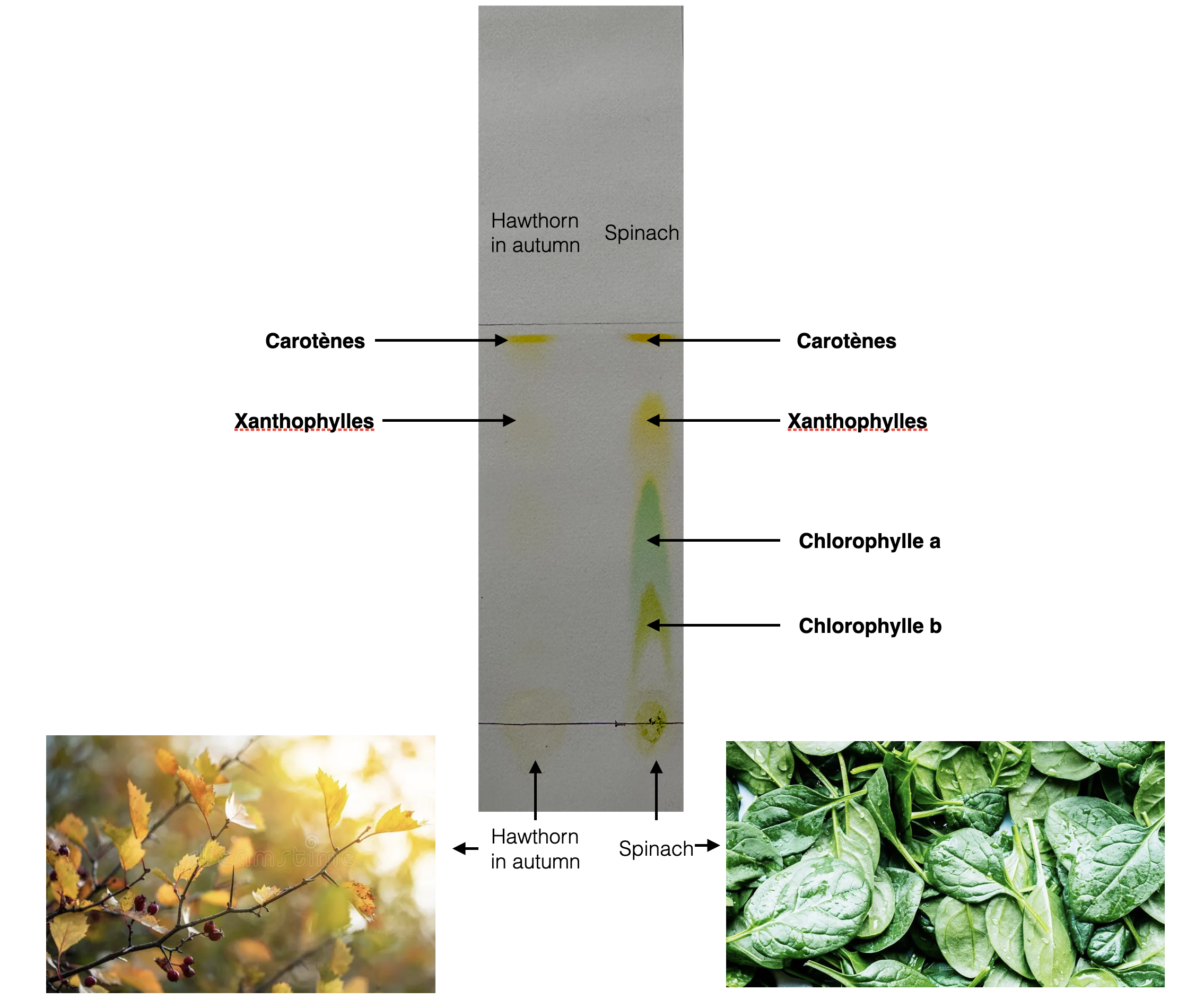
J. Boscq
Results and analysis
- Do the two leaves have the same pigments?
- If they have, is there a difference in quantity of some pigments?
- Calculate the RF Value of each pigment of your chromatography for both of your leaves
- Compare it with the official Rf Values just below. Is there any differences?
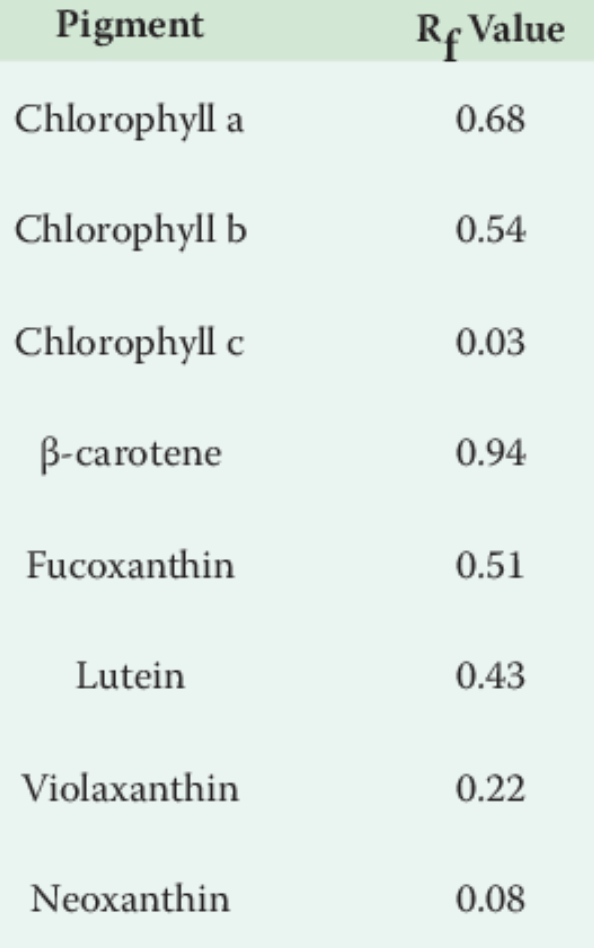
5. The spinach leaf looks green, but your chromatogram demonstrated that other pigments are in the leaf as well. Why can you not readily see the other pigments in the leaf?
6. The maple leaf is red in automn, but your chromatogram demonstrated that other pigments are also in the leaf. What is the difference between the pigments in spinach leaves and maple leaves?
7. Would you expect a plant to grow well in only green light? Explain.
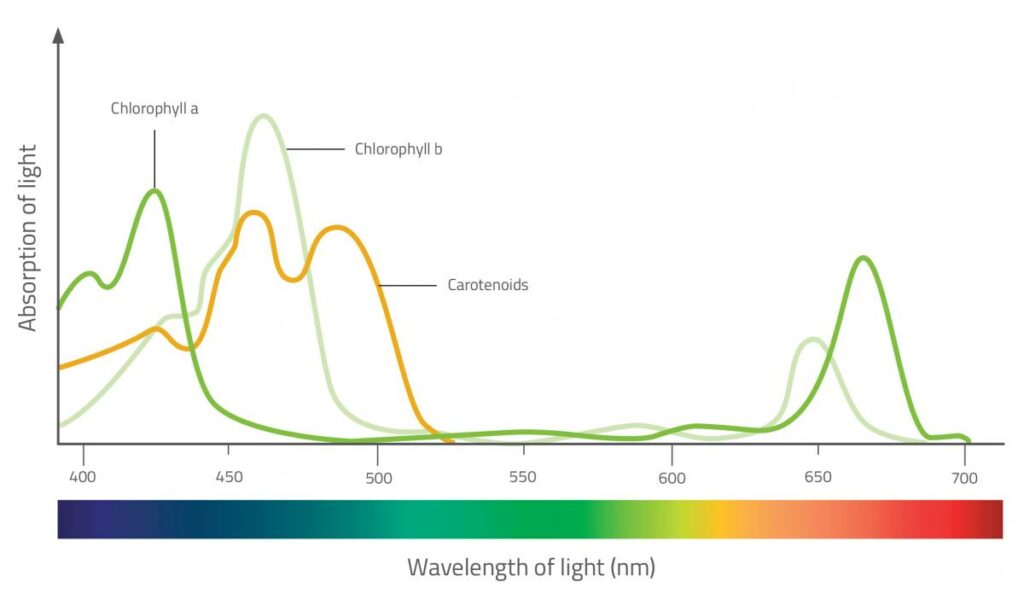
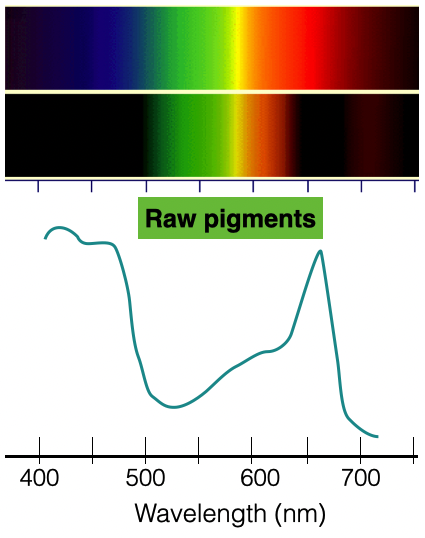
8. What does the absorption spectrum convey about each pigment?