Text analysis: Symbiosis of the flatworms of Roscoff
A- abstract

A remarkable example of biological engineering is the capability of some marine animals to take advantage of photosynthesis by hosting symbiotic algae. This capacity, referred to as photosymbiosis, is based on structural and functional complexes that involve two distantly unrelated organisms. These stable photosymbiotic associations between metazoans and photosynthetic protists play fundamental roles in marine ecology as exemplified by reef communities and their vulnerability to global changes threats. Here we introduce a photosymbiotic tidal acoel flatworm, Symsagittifera roscoffensis, and its obligatory green algal photosymbiont, Tetraselmis convolutae (Lack of the algal partner invariably results in acoel lethality emphasizing the mandatory nature of the photosymbiotic algae for the animal’s survival). Together they form a composite photosymbiotic unit, which can be reared in controlled conditions that provide easy access to key life-cycle events ranging from early embryogenesis through the induction of photosymbiosis in aposymbiotic juveniles to the emergence of a functional “solar-powered” mature stage. Since it is possible to grow both algae and host under precisely controlled culture conditions, it is now possible to design a range of new experimental protocols that address the mechanisms and evolution of photosymbiosis. S.roscoffensisthus represents an emerging model system with experimental advantages that complement those of other photosymbiotic species, in particular corals. The basal taxonomic position of S. roscoffensis (and acoels in general) also makes it a relevant model for evolutionary studies of development, stem cell biology and regeneration. Finally, it’s autotrophic lifestyle and lack of calcification make S. roscoffensis a favorable system to study the role of symbiosis in the response of marine organisms to climate change (e.g., ocean warming and acidification).
The chimerical and multifaceted marine acoel Symsagittiferaroscoffensis:fromphotosymbiosistobrain regenerationXavier Bailly
B- an extract

S.roscoffensisis a gregarious intertidal symbiotic acoel endemic to the North Atlantic coasts. Around 4 mm in length, it lives interstitially and is found during daylight on the upper part of beaches in calm residual flows on ebb tides. The number of algal cells within adult S. roscoffensis individuals has been estimated to be around 40,000 (Doonan and Gooday, 1982). Up to millions of individuals congregate and form mats in more or less discrete patches notably where exposed to the sunlight that favors photosynthetic activity of the photosymbiotic algae.
Juveniles ingest environmental free algae within the first few days after hatching. Algae are taken up into a vacuole in the syncytial central parenchyme where they lose their cell wall (theca), the four flagella and the eyespot (light receptor), resulting in a significantly modified phenotype
Colorless aposymbiotic juveniles must encounter free-living T. convolutae cells in order to initiate the obligate symbiosis and develop directly without intermediate larval stages. If present, they can ingest algae naturally occurring in seawater otherwise algae can also be supplied from controlled laboratory cultures
After natural or artificial induction of symbiosis, aposymbiotic juveniles become fully green within 10–15 days
whereas worms that remain aposymbiotic decline progressively and do not survive for more than 20 days
Captive algae produce photosynthetates, such as mannitol and glutamic acid, which are at the origin of the synthesis/release of amino acids by the worm
On hatching, juveniles exhibit solid uric acid that increases during the aposymbiotic state but decreases gradually to undetectable levels once the association with the algae take place
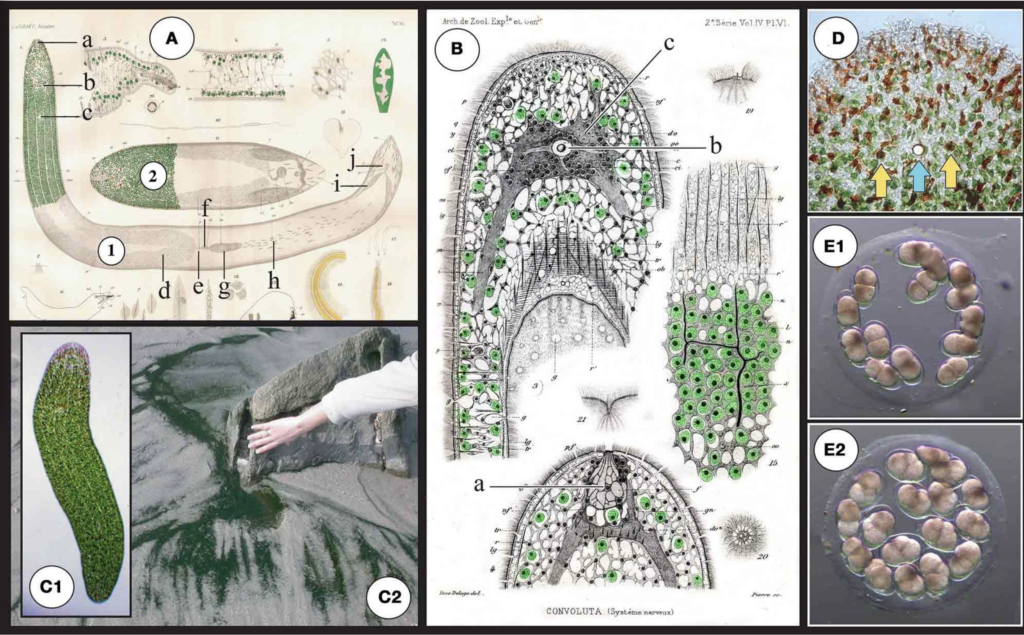
S. roscoffensis (1) together with a closely related (dwarf) species S.schultzii neural bundles. (C2)Dark green mat on the beach: a typical colony of S.
(ex Convolutaschultzii) (2)and in particular the localization of algae in the roscoffensis in the residual flows on ebb tides close to Roscoff, Brittany, epidermal area. a: frontal organ (sensing), b: statocyst c: mouth, d: ovocyte, e: France. (D) Magnification of the anterior part. Note the reduced presence of female genital pore, f: bursal nozzle [the bursa nozzle leads from the seminal algae surrounding the anterior pole and the occurrence of reddish structures; bursa (g) to the female genital opening (e)], g: seminal bursa, h: sagittocysts, i: these rod-shaped epidermal mucus secretion bodies are called rhabdoids seminal vesicles, j: male genital pore. (B)Original drawings from Delage (1886) (Smith et al, 1982). Yellow arrows show the two photoreceptors flanking the a: frontal organ (sensing), b: (statocyst) c: central nervous system (gray area central statocyst involved in gravity sensing (the blue arrow). (E)Egg capsule surrounding the statocyst). (C1) Adult symbiotic S. roscoffensis(see also (cocoons) with early developmental stage embryos (E1) 2-cell stage with two magnification of the anterior pole in D). Note the presence of four visible lines macromeres. (E2) 4-cell stage: first duet of micromeres.
1) Try to observe on the Roscofensis worm all the details which are in this article. And in the same time translate all the words that you don’t know.
2) Demonstrate your understanding of the main ideas and important details of each text above.

